For most of the past decade, the number of drugs approved every year per dollar spent on R&D has remained flat or decreased. The expenses for a newly approved drug will reach unexpected values over the next few years if this trend continues. (see What’s Eroom’s Law?) (1). Could the re-use of old drugs for new applications revert this trend?
Drug Repurposing is the strategy of reusing an existing drug or compound candidate (which has already been proved as safe) to treat a different disease or disorder (2).
The starting point for repurposing can be:
- Approved drugs. Marketed drugs have been approved by FDA so their safety profiles are well defined. That makes easier and cheaper to achieve late clinical phases against new indications (Clinical Phases II and III), therefore becoming really attractive targets for pharma companies. Approved drugs treating a new disease can still be covered by patents or granted by FDA. (3)
- Failed drugs and drugs under development. Some candidates were never approved after failing at Phase II or III. If these candidates are reprofiled, it won’t be necessary to go again through preclinical development and Clinical Phase I, but only tested from Human Proof of Concept phase onwards. Similarly, a drug under development might be reprofiled provided that Clinical Phase I has been successfully completed. Both strategies yield, highly increased probabilities of success. (3)
In either case, repurposing will provide the following advantages:
- Achieving FDA approval in a shorter time
- Decreasing the R&D costs
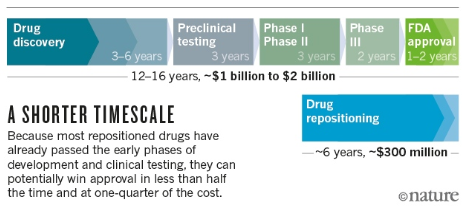
Drug repurposing (also called drug repositioning), will decrease the time and the cost of the drug development process. Image obtained from Nicola Nosengo, Can you teach old drugs new tricks?
Several classic repurposing success stories have been described. One of them is Thalidomide, which was first used as a sedative for pregnants, but it was withdrawn due to undesirable side effects in the fetus. Nowadays, Thalidomide has shown efficacy in the treatment of some multiple myeloma cancers that don’t respond to classical chemotherapy. Another curious example is Sildenafil, which was first used in cardiovascular diseases, but now is widely marketed for treating erectile dysfunction (Viagra) (3,4).
Several repurposing approaches are being explored. Some of these strategies are HTS, which implies testing a huge amount of candidates against different cell lines; Big Data Analytics, that can uncover molecular similarities between diseases and Activity Prediction, that can predict which compounds might take advantage of similarities based in computational models, among others.
How field-based virtual screening contributes to drug reprofiling?
Computational chemistry tools are especially valuable when looking for already existing assets to be applied for new indications. Ligand-based approaches can be used to virtually screen libraries of molecules against known active chemical entities. For Drug Repurposing, subsets composed by either approved drugs, failed or existing candidates (if Phase I has been successfully completed) might be used as such libraries. Virtual resulting hits might subsequently enter Human Proof of Concept phases.
The use of 3D descriptors has been described as an optimal ligand based technique to find such new applications for either existing drugs or failed or existing candidates.
Pharmacelera’s technology uses a unique 3D representation of molecules based on electrostatic, steric and hydrophobic interaction fields derived from semi-empirical Quantum-Mechanics (QM) calculations. This allows a more accurate exploration of the chemical space.
Are you working on drug repurposing? Do you want to know more about how our technology can help you increase your R+D productivity in this field? Contact us for an open discussion with no commitment!
If you want to learn more, you can find further information at:
(1) Jack W. Scannell, Alex Blanckley, Helen Boldon, Brian Warrington, “Diagnosing the decline in pharmaceutical R&D”, Nature Reviews Drug Discovery, vol 11, 191–200, 2012
(2) Joris Langedijk, Aukje K.Mantel-Teeuwisse, Diederick S.Slijkerman, Marie-Hélène, D.B.Schutjens, “Drug repositioning and repurposing: terminology and definitions in literature”, Drug Discovery Today, Vol 20, Issue 8,1027-1034, August 2015, https://doi.org/10.1016/j.drudis.2015.05.001
(3) Nicola Nosengo, “Can you teach old drugs new tricks?” Nature 534, 314–316, June 2016, doi:10.1038/534314a
(4) Anna Azvolinsky, “Repurposing Existing Drugs for New Indications”, The Scientist, Jan 2017
