By Giorgia Zaetta – Dec. 11, 2020
Lipophilicity is the property of a molecule that describes its distribution between water and octanol, and it is the expression of the intermolecular forces that dictate the solvation in the aqueous and organic phases. Lipophilicity and hydrophobicity are often used as equivalent concepts; however, they are not strictly synonymous, the latter being in fact one of the contributor factors to the first. The lipophilicity of a molecule results from the balance of hydrophobic field (partition of the lipophilic phase), and polar/ionic features (partition of the aqueous phase through energetically favourable solvation by water). The hydrophobicity term describes hydrophobic and Van der Waals forces, while the polarity term accounts for hydrogen bonding, orientation, and inductive effects.
Effect of Lipophilicity in Drug Potency
Ligand lipophilicity impacts target affinity since most druggable binding sites contain at least one hydrophobic pocket in a surrounding aqueous environment. The hydrophobic effect drives interaction of ligands to the protein binding sites via replacement of interactions between the protein and solvating waters with more favourable hydrophobic interactions for both ligand and protein (1).
Effect of Lipophilicity in PK Parameters
There is an optimal range of lipophilicity to achieve “drug-likeness” because in addition to binding affinity, lipophilicity has effects on other molecular attributes. Highly soluble compounds possess poor permeability through biological membranes, limiting absorption along the gastrointestinal (GI) tract or the transport across the blood brain barrier (BBB). Overall, a careful handling of lipophilicity is required to optimize compound availability at the biological target. Variation of lipophilicity within a series of designed compounds can predictably affect both potency and ADME properties (2).
Effects of Lipophility in Drug Discovery
The lipophilicity of molecules can be tailored by design through controlling the balance of hydrophobic features. This concept has been accompanied by the progressive variety of in silico techniques to exploit the pharmacophore concept. This is exemplified by virtual screening (VS) studies of large molecular databases performed to identify new promising compounds according to their similarity to a reference that should contain physicochemical features relevant for biological activity. A sensitive and effective estimation of molecular similarity is a fundamental pre-requisite for the identification of potential leads starting from a chemical reference, which represents the paradigm of VS (3).
Conclusion
In conclusion, there appears to be a consensus on the importance of balancing properties in drug design. However, we believe that oversimplified strategies with biases for individual parameter ranges and/or directionality for parameter improvement are less efficient at achieving this. While the impact of lipophilicity on individual parameters is well documented in the literature and generally well-embraced, the extent to which these effects tend to offset when fully integrated into the resulting efficacious dose seems to be underappreciated.
This is the challenging and complex nature of drug design: the need to optimize many parameter values relative to each other. While these strategies can appear more complex and difficult to execute, in our experience they are also more likely to result in both improved success and enhanced efficiency in drug discovery.
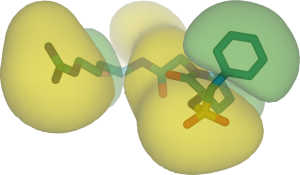
PharmScreen® uses a unique and superior 3D representation of molecules based on electrostatic, steric, and hydrophobic interaction fields derived from semi-empirical Quantum-Mechanics (QM) calculations. Such fields describe with high accuracy the factors that determine ligand / receptor interactions.
Do you want to find out more about how hydrophobic interactions can be useful in your drug discovery project? Let us help you! Our consultants will provide you with customized solutions and always working side by side with your team. Do you want to know more about our services and technologies?
Contact us (contact@pharmacelera.com) for an open discussion and a free trial of PharmScreen.
Bibliography
(1) “Lipophilic Efficiency as an Important Metric in Drug Design” Ted W. Johnson, Rebecca A. Gallego, and Martin P. Edwards, J. Med. Chem. 2018, 61, 6401−6420
(2) “Integrating the Impact of Lipophilicity on Potency and Pharmacokinetic Parameters Enables the Use of Diverse Chemical Space during Small Molecule Drug Optimization” Randy R. Miller, Maria Madeira, Harold B. Wood, Wayne M. Geissler, Conrad E. Raab, and Iain J. Martin, J. Med. Chem. 2020, 63, 21, 12156–12170
(3) “Lipophilicity in drug design: an overview of lipophilicity descriptors in 3D-QSAR studies” Tiziana Ginex, Javier Vazquez, Enric Gilbert, Enric Herrero, Francisco J Luque, Future Medicinal Chemistry 2019 Vol. 11, No. 10